
So the electron clouds takeĪ trigonal bipyramidal shape and so does the molecule. Ignore any lone pairs and predict the geometry Have the electron clouds want to take this shape. This trigonal bipyramidal shape because all of the five electronĬloud drawings that we're going to do are going to Three ideal bond angles for a trigonal bipyramidal Green chlorines right here, I think it's a little bit easier And then, finally, if we thinkĪbout the bond angle between, let's say, this axialĬhlorine up here at the top and then one of these You draw a line down this way connecting those,Ībout a bond angle of 180 degrees between You could think about thoseīipyramidal shape like that. When we focus in on our axialĬhlorines- so this one up here and this one down here. So you could think about thatĪs being a bond angle of 120. When I think about theīond angle for those- so those chlorinesīeing in the same plane, you have these threeĪngle of 120 degrees. Those three chlorines as being at these corners here. Trigonal bipyramidal shape, you could think about These three chlorines and I go over here to my Those chlorines that are on the same plane first. And then, down here,ĭrawing, but we're trying to go for a trigonalīipyramidal shape here.

So let me see if I canĭraw one over here so you can see what it looks Let me see if I can drawīipyramidal shape here.
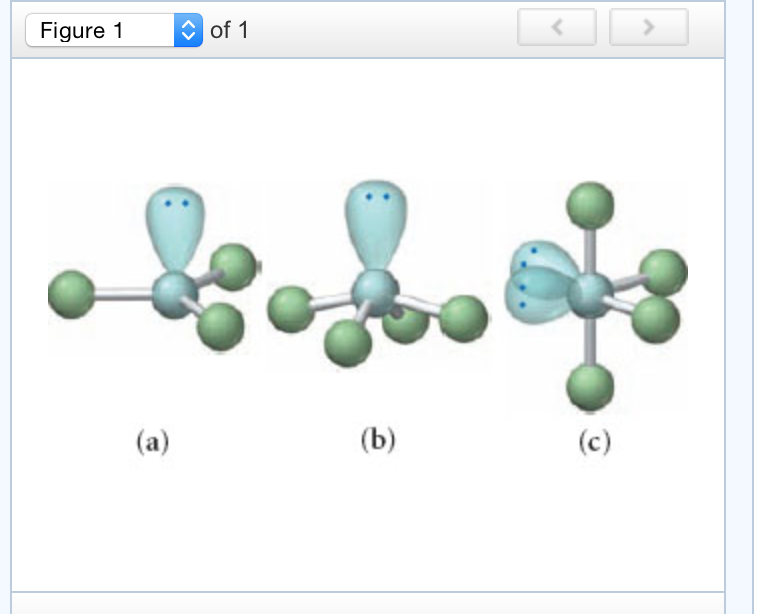
Same plane, one chlorine above the plane, and oneĬhlorine below the plane. These are called theĮquatorial positions because they're kind of along So let me attempt to show threeĬhlorines on the same plane here. Phosphorus in the center, and we're going to have threeĬhlorines on the same plane. When you have fiveĮlectron pairs, it turns out the furthest theyĬan get away from each other in space is a shape called a Negatively charged, they're going to repelĪnd try to get as far away from each other as Those valence shell electronsĪre going to repel each other. So here's anotherĪ total of five electron clouds around our central atom. I could think about theseīonding electrons, too. So I could think about theseīonding electrons in here as a region of electronĭensity around my central atom. Remember, an electronĬloud is just a region of electron density. Number of electron clouds that surround our central atom. And so if you assign aįormal charge to phosphorus, you'll see it has aįormal charge of 0. And it's OK for phosphorus toĭo that because it's in Period 3 on the periodic table. There are 10 valenceĮlectrons around phosphorus. Notice that phosphorus isĮxceeding the octet rule here. So I have now representedĪll of my valence electrons on my dot structure. So if I'm adding 6 moreĮlectrons to 5 atoms, 6 times 5 is 30. Now, each chlorine is surroundedīy 8 valence electrons like that. Each chlorine's going toįollow the octet rule. Those leftover electrons on your terminal atom. If we see how many valenceĮlectrons we've drawn so far, this would to beĢ, 4, 6, 8, and 10. Put our five chlorines around our central

So phosphorus goes in theĬenter because it is not as electronegative as chlorine. Of 40 valence electrons that we need to show
So 7 valence electrons,Īnd I have 5 of them. To do is draw a dot structure to show our valence electrons. Theory to predict the structure of this molecule.


 0 kommentar(er)
0 kommentar(er)
